Abstract
Background/Introduction: The family of B-cell lymphoma-2 (Bcl-2) proteins plays an important role in multiple myeloma (MM) cell survival and represents an attractive therapeutic target. In prior trials, the combination of a Bcl-2 inhibitor, a proteasome inhibitor, and dexamethasone, showed increased mortality in the ITT population, but a subgroup analysis of patients with t(11;14) positive R/R MM showed an improved progression free survival, with no increased mortality. Compared to venetoclax, BGB-11417 is a more potent (>10 fold in biochemical assays) and highly selective Bcl-2 inhibitor.
The ongoing BGB-11417-105 trial (NCT04973605) is a phase 1b/2 study determining the safety and efficacy of BGB-11417 as monotherapy, in combination with dexamethasone, or with dexamethasone plus carfilzomib in patients with t(11;14) positive R/R MM. Here, we present preliminary safety results for the combination of BGB-11417 and dexamethasone from study BGB-11417-105.
Methods: Eligible patients with t(11;14) positive R/R MM who had been exposed to a proteasome inhibitor, immunomodulatory agent, and an anti-CD38 therapy were included. Patients received 80, 160, 320, or 640 mg of BGB-11417 daily with 40 mg of dexamethasone weekly until death, intolerability, or disease progression. Adverse events (AEs) were reported per Common Terminology Criteria for AEs v5.0. Dose escalation (after a 21-day dose-limiting toxicity window) was guided by a mTPI-2 design and overall review by a safety monitoring committee. Pharmacokinetics (PK) was also assessed.
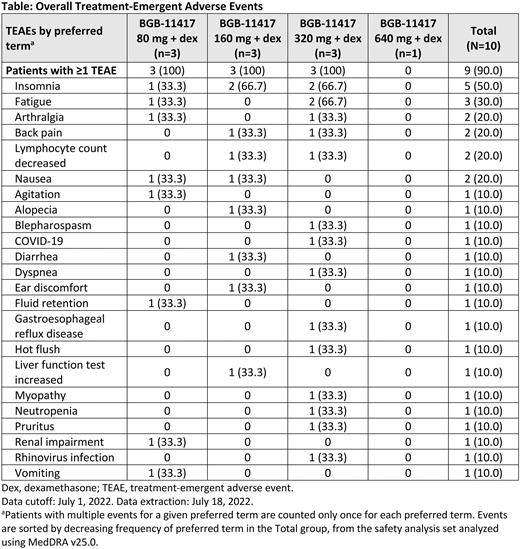
Results: As of the data cutoff date, July 1, 2022, a total of 10 patients have been enrolled in the 80, 160, and 320 mg (3 patients each) and 640 mg (1 patient) dose-escalation cohorts of BGB-11417 plus dexamethasone. The median age of all patients enrolled in the trial was 69 years (range, 52-81), median prior lines of therapy was 3 (range, 1-5), 7 patients had an ECOG performance score of 0, and 3 patients had a ECOG score of 1. The median treatment duration was 3.2 months (range, 0.5-6.5). No patients experienced dose-limiting toxicity at any dose level tested. Three patients died while on study, 1 due to COVID-19 complications 157 days after treatment discontinuation on day 208 of the study, 1 due to progressive disease 50 days after treatment discontinuation on day 89 of the study, and 1 due to COVID-19 while on study treatment at day 78. All deaths were determined to not be associated with study treatment, by the investigator. Two other patients experienced grade ≥3 treatment-emergent AEs (TEAEs). One patient in the 160 mg cohort experienced a grade 3 increase in liver enzymes and lymphopenia. One patient in the 320 mg cohort experienced a grade 3 lymphopenia. The most common TEAEs were insomnia (50%), fatigue (30%), arthralgia (20%), back pain (20%), lymphopenia (20%), and nausea (20%). A summary of TEAEs observed for each dose level is shown in the Table.
BGB-11417 exposure increased in a dose-dependent manner from 80 mg to 320 mg with high inter-patient PK variability. BGB-11417 exposures after single and multiple doses appeared similar, indicating limited accumulation of BGB-11417.
Conclusion: BGB-11417 in combination with dexamethasone was generally well tolerated in patients with R/R MM harboring t(11;14) at doses up to 640 mg. Efficacy data are forthcoming. Recruitment is ongoing in the US, Australia, and New Zealand; the BGB-11417, dexamethasone, and carfilzomib combination arm will open in the future.
Disclosures
Quach:Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: receipt of free drug for investigator-initiated study, Research Funding; CSL: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role, receipt of free drug for investigator-initiated study , Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role , Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role, receipt of free drug for investigator-initiated study , Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Antengene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: receipt of free drug for investigator-initiated study, Research Funding. Rajagopal:CSL: Current equity holder in publicly-traded company; Janssen: Honoraria. Spencer:Haemalogix: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria. Low:Janssen: Consultancy; BMS: Honoraria. Kazandjian:BMS: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Arcellx: Membership on an entity's Board of Directors or advisory committees; MMRF: Honoraria; Curio Science: Honoraria; Aptitude Health: Honoraria; Plexus Communications: Honoraria; SINTOMA: Honoraria; CURE: Honoraria. Crescenzo:BeiGene: Current Employment, Current equity holder in publicly-traded company; Pfizer: Current equity holder in publicly-traded company; GSK: Current equity holder in publicly-traded company; SAGA Diagnostics: Current equity holder in private company. Du:BeiGene: Current Employment, Current equity holder in publicly-traded company. Patel:BeiGene: Current Employment, Current equity holder in publicly-traded company. Mundra:BeiGene: Current Employment, Current equity holder in publicly-traded company. Cheng:BeiGene: Current Employment, Current equity holder in publicly-traded company. Dhakal:BMS: Honoraria, Research Funding; Karyopharm Therapeutics: Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Research Funding, Speakers Bureau; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Natera: Consultancy; Arcellx: Research Funding; Carsgen: Research Funding; Cartesian: Research Funding; Fate: Research Funding; Takeda: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.